Uncategorised
Are biostimulators the future?
Being a Key Opinion Leader and being part of the faculty for Allergan Aesthetics, means I get to have a front-row seat when it comes to innovation in injectables.
This role has been one of the nicest surprises career-wise in the last year and is something I really enjoy. It has seen me travelling to places like Brazil, Monaco and Berlin to speak in front of my peers, being asked to be the principal investigator on a Phase 3 clinical trial for the use of toxin in the lower face and masseter. As well as this, I was invited to be one of a handful of practitioners appointed to the faculty for the company’s new hybrid dermal filler – HArmonyCa with Lidocaine.
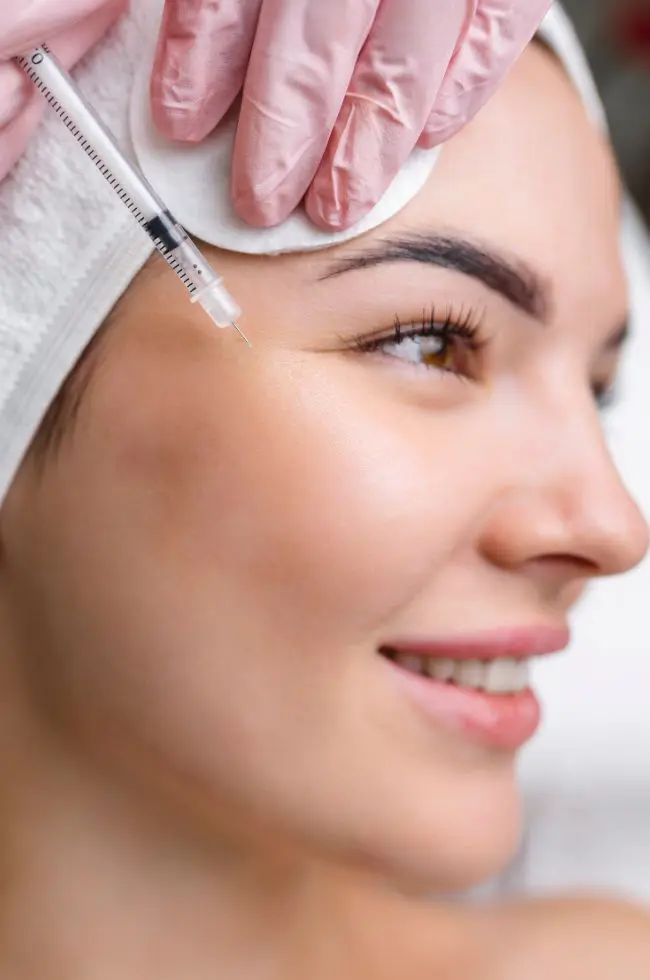
HAarmonyCa is Allergan’s first dual-effect product formulated for facial soft tissue augmentation via injection into deep dermal and sub-dermal layers of the skin. It combines two active ingredients – hyaluronic acid (HA) and calcium hydroxyapatite (CaHA) – in one injection, making it an exciting new option for patients and aesthetic practitioners alike. This is because it answers the multi-dimensional needs of ageing skin in one treatment. You get the immediate lifting effect from the HA combined with sustained lifting from the CaHA, which is known to help stimulate collagen. Loss of facial volume and changes to the soft tissue are two of the most common signs of ageing. Global research exploring people’s attitudes to ageing and aesthetics showed almost all respondents (96%, N=12,360) had a concern about an area of their face, with more than a third stating they were concerned about the quality of their skin (40%, N=12,360)or its loss of tightness (39%, N=12,360).1
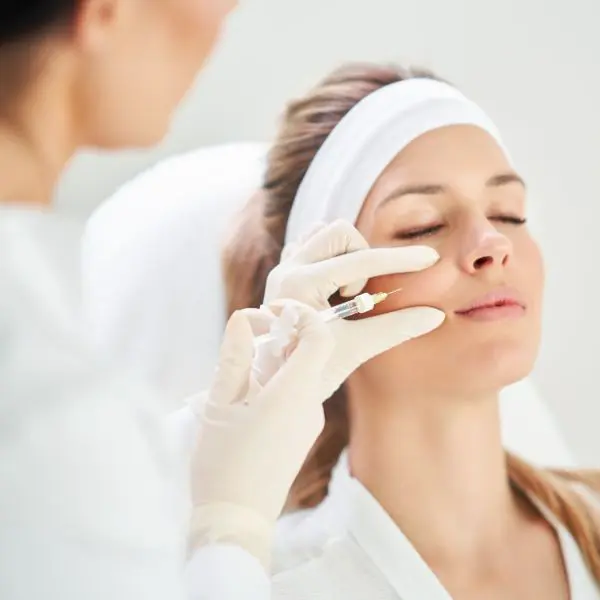
HAmonyCa Study
In a recent clinical study of HAmonyCa, 86% of 85 treated regions among 48 patients showed clinically significant improvement, with over 90% of patients registering an improvement in the general appearance of facial areas treated. The study also demonstrated that the product has a low risk-benefit profile.2
The majority of patients reported high user satisfaction (mean 4.1 out of 5) for overall satisfaction and likeliness to repeat similar treatment up to 19 months after receiving HArmonyCa.
References
1. Consumer Beauty Insights Study 2021 – REF-83962
2. Allergan Aesthetics. Data on File. INT-HAR-2150007. HArmonyCaTM. Clinical Study Report. April 2021.)
HAmonyCa
One of the reasons I am most excited about HAarmonyCa is that it sits in the category of injectables known as “biostimulators”, something I believe is going to become more and more prevalent in the future of aesthetic medicine. Unlike regular dermal fillers, biostimulators stimulate new tissue growth, helping the skin to produce its own volume naturally. What I like about them, and what makes them popular with patients, is the way they can rejuvenate in such a natural way.
Patients like the idea that their own bodies are contributing to rejuvenation. Some patients have been put off of dermal fillers because of things they see in the media, and, for new patients, biostimulators can be like a gateway treatment.
At Interface Aesthetics, we pride ourselves on offering the highest level of education, and therefore, this must include the use of new and cutting-edge techniques that are shaping the industry. As such, we are delighted to announce we have launched a new biostimulators course to. be held on 16th July 2023.
Like our other courses, this will be evidence-led and will teach delegates about the clinical uses and techniques for delivering this evolving category of treatments. More on masterclasses here.
